Dry Flocculant Mixing and Feeding Design Considerations
This is a guest post by Bill Hancock, President of Zeroday Enterprises
Dry Flocculant Mixing and Feeding Design Considerations
Dry flocculant wetting, dissolving, mixing and solution feeding must be done under very specific controlled conditions that account for the flocculant’s unique physical and chemical characteristics. Failure to ensure proper polymer solution preparation and feeding can result in mix-feed system plugging and erratic operation, process control issues and wasted polymer.
Key flocculant characteristics that must be considered for proper flocculant mixing and use are summarized below:
1) Flocculant powders are hydroscopic. Storage and dry polymer feeding systems must ensure minimum air contact as the particles will adsorb moisture which initiates flocculant dissolution. Dissolving particles are very sticky and consequently will aggregate into larger pieces and chunks. These larger pieces and chunks dissolve slower and often can plug equipment causing mix system and even process operational disruptions.
2) Water wetted flocculant particles can aggregate into masses. Since flocculant particles dissolve proportionately to exposed surface area, particle aggregates that have stuck together result in significantly reduced dissolution rate during mixing. If the flocculant aggregates do not fully dissolve within the set system residence time, these partially flocculant masses will pass through the mix system and process unused. And possibly plug equipment. Note: flocculant particles/aggregates will hydrolyze throughout into a clear gel which refract light differently than water, these masses are called ‘fish eyes’ because can predominantly see the light refraction, not the mass itself.
3) It is very important to initially wet each flocculant particle. The mixing system must be designed in the first step of the mixing process to wet each particle individually and immediately, and subsequently ensure these wetted particles do not aggregate or agglomerate. There are two main mix system flocculant particle wetting categories.
- Augering flocculant particles into a funnel with plenty of water flowing in the funnel followed by some motive dispersive force to ensure any particles that might stick together will be pulled apart.
- The flocculant particles are air blown from an auger into a large diameter hose to the top of the mix tank where cascading water inside a tube wets the particles which enters the mix tank and impeller.
4) Flocculant dissolution time variable. Flocculants dissolve at different rates, primarily dependent on the following factors.
- Type of polymer charge
- Flocculant charge level
- Water temperature and chemistry
Required mix times can range from 30-90 minutes are designed into a mix system.
5) Flocculant solutions are viscous. Dissolved long chain flocculants introduce solution drag that is expressed as viscosity. The amount of viscosity is dependent on the flocculant charge, charge level, temperature, concentration and molecule chain length. More viscous solutions require more mixing power. To ensure the flocculant molecule chains are not broken, balancing the amount of power in mixing and the type of mixing are crucial for minimizing dissolved flocculant chain damage.
6) Low shear pumps must be used for flocculant solution transfer and feeding. The two more predominate low shear pump types are progressing and diaphragm. More specifically, examples of pumps that are acceptable include progressive cavity, peristaltic, gear, lobe and air operated diaphragm.
7) Flocculant solutions typically provide best performance at ≤ 0.1% concentrations. To optimize flocculant mixing system sizing, flocculant solutions are often designed to be mixed to 0.25-0.50% maximum concentration. Once dissolved, the flocculants will dilute readily with teed in water down stream of the flocculant feed pump to obtain the final target 0.1% concentration.
Flocculant mixing and feeding is not highly complex. But the unique flocculant characteristics accounted and designed into a mixing-feeding system to ensure optimum preparation and minimized consumption.
Bill Hancock is an internationally recognized expert in mineral processing technologies, technical marketing management and water treatment. Hancock founded and owns Zeroday Enterprises which supplies chemical mix-feed systems, LTM conductive slurry level monitoring probes, peristaltic hose and tube pumps, mixers and flocculant and coagulant chemicals. He also founded Argo Consulting—a technical and technical marketing consulting practice focused on providing mineral processing, water treatment and technical marketing consulting services to the mining industry.

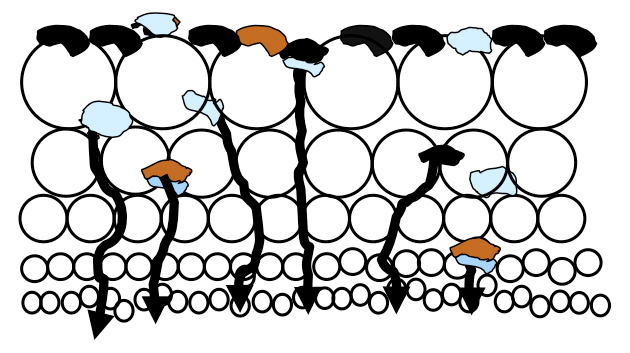
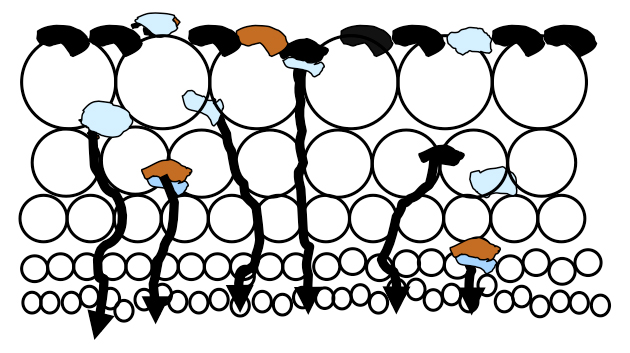 There is a massive amount of air in a flotation cell, typically 12-25% of a float cell’s total volume, which rises enmass to the froth layer as fine bubbles. Much slurry is pushed into the lower froth bed which is held in the voids between the bubbles until the slurry can drain back into the cell. As illustrated in the figure, there is more slurry in the lower froth bed than higher due to drainage.
There is a massive amount of air in a flotation cell, typically 12-25% of a float cell’s total volume, which rises enmass to the froth layer as fine bubbles. Much slurry is pushed into the lower froth bed which is held in the voids between the bubbles until the slurry can drain back into the cell. As illustrated in the figure, there is more slurry in the lower froth bed than higher due to drainage.